Your Cart is Empty
Menu

The Easiest Way to Draw a Cyclohexane Chair Conformation and Ring Flip
4 min read
Drawing the cyclohexane chair conformations adds unnecessary stress to most chemistry students. We've created a hack to draw the cyclohexane chair and boat conformations perfectly every time.
Follow the steps below to easily draw the chair conformation and ring flip like a pro so you can focus your mental energy on solving the reaction itself.
Step 1: Draw two parallel horizontal lines slightly offset.
The upper line should be shifted to the left so that it halfway overlaps the lower line. I suggest half inch or roughly 1.2 cm long lines. It's easiest to draw them parallel to your notebook paper lines when you're a beginner.
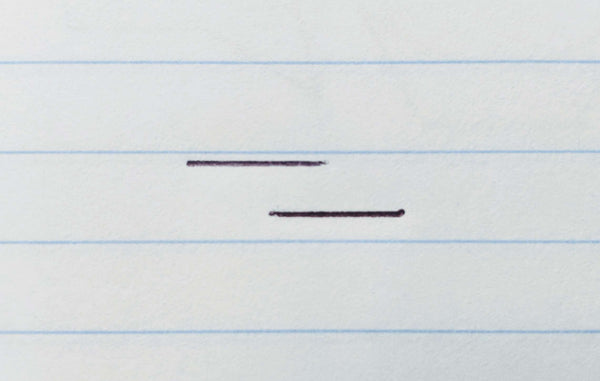
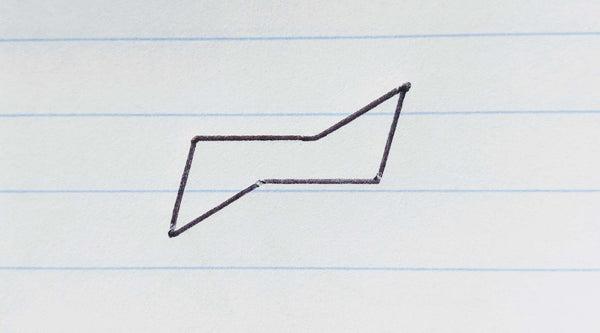
Step 2: Place a dot for each pointy end of the ring and connect it to your lines.
Imagine an isosceles triangle where the base connects the two nearest ends of each straight line. Add a dot where the point of the triangle would go.

Connect your dot to the two nearest ends of the parallel lines.
Repeat this for the other end to complete your hand-drawn chair conformation! Scroll down for how to draw axial and equatorial bonds to your chair conformation.
If yours looks a little sloppy, consider grabbing The Pocket Chemist molecule stencil below to draw quickly draw your chair and boat conformations, benzene rings bonding angles perfectly every time. Get the drawing done in a third of the time so you can focus on the difficult parts of the reactions.
Alternate Step 2: Use The Pocket Chemist molecule stencil
Use the chair conformation stencil on The Pocket Chemist. Decide which ring flip you need to draw. The most traditional representation is drawn with the highest point at the top right which is drawn with the periodic table side of the pocket card facing up.
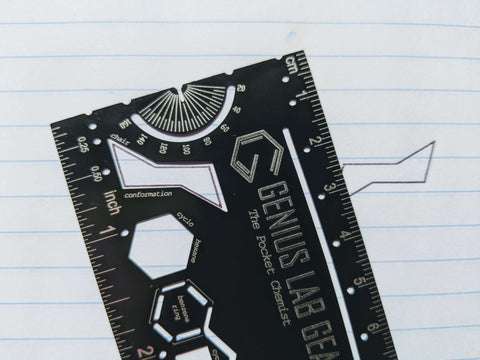
If you don’t have it yet, The Pocket Chemist is available for purchase from our store with free shipping in the USA or on Amazon using the link below! It’s been endorsed by chemistry teachers, chemistry professors, professional chemists and organic chemistry tutors from around the country.
It’s designed to help you quickly and neatly draw chemical reactions for both homework and professional work while also providing references for fundamental equations, physical constants and unit conversions in every-day chemistry. The stencil will also help you draw boat conformations, benzene rings, carbon chains, cyclopropane, cyclobutance and cyclopentane!
Step 3: Draw the axial position bonds
The axial position bonds should go first. They alternate up and down at each carbon as shown by the blue lines in the image below. If you had The Pocket Chemist facing down (showing the periodic table), start by drawing an “up” bond at the top right corner and a “down” bond on the bottom left corner, with the lines exactly vertical.
Use the straight edge of The Pocket Chemist to keep your lines straight and measure each line length to 5 mm to keep things proportional. Finish by filling in alternating “up” and “down” axial position bonds moving around the cyclohexane plane of the ring.
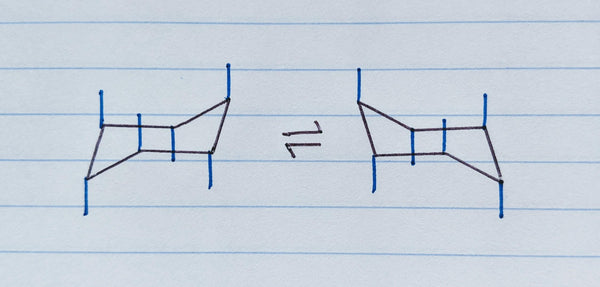
Step 4: Draw the equatorial position bonds:
Now add equatorial position bond lines in the opposite direction (up or down) of each axial position bond. These should not be perfectly opposite the axial bonds nor at a perfect 90 degree angle, as shown by the red lines below.
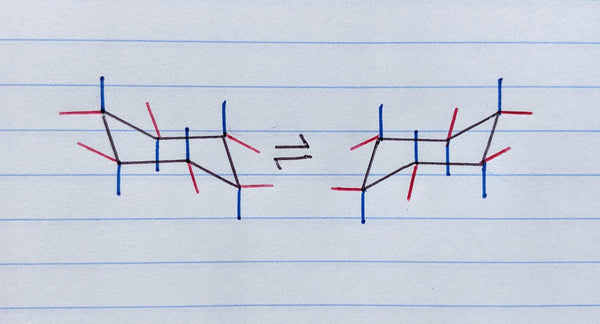
The easiest way to draw equatorial bonds on the chair conformation is to make them parallel to the cyclohexane ring line that is the second line moving clockwise or counter-clockwise around the ring plane from the carbon you’re drawing the equatorial bond on (see the highlighted lines below).
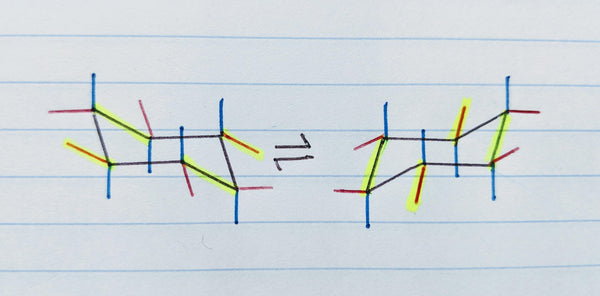
You can align the straight edge of The Pocket Chemist to one of the existing lines then slide it into position to make your equatorial position bond perfectly parallel. This applies for all six equatorial bonds and in both cyclohexane ring flip conformations!
Step 5: Draw a ring flip chair conformation
The ring flip chair conformation (or chair flip) is mirrored across either a horizontal or vertical line on your paper. If you have The Pocket Chemist, simply flip it over. You don’t need to worry about the rotation since it has rotational symmetry.
Remember the golden rule: Up bonds stay up and down bonds stay down, but axial bonds become equatorial bonds and equatorial bonds become axial.
Remember that axial bonds should be drawn vertically and equatorial should be neither vertical nor horizontal, parallel to the 2nd line clockwise or counter-clockwise from the carbon you’re drawing the bond on.
Step 6: Add your molecules to the cyclohexane chair conformation (if required)
When adding your molecules such as a methyl group, it’s much easier to see details at the four corners. Avoid attaching complex molecules to the two middle carbons in the chair conformation. Remember that substituents larger than hydrogen begin to have steric crowding and steric strain when they are axial on a chair conformation rather than equatorial. Substituted cyclohexanes will prefer more stable conformations where the larger substituents assume equatorial orientations.
Draw molecules faster and more clearly using The Pocket Chemist
We designed this popular tool specifically to help chemists and chemistry students get past the frustrating task of drawing molecular shapes and get on with the real work of studying the reactions. It fits in your wallet, purse or pocket right behind a credit card or ID so you can always have it ready.
We also took the liberty of packing the free space with a full laser-engraved periodic table, physical constants, fundamental equations, metric and imperial rulers and an angle measurement tool. The enamel-coated stainless steel will stand up to years of abuse.
To see the full list of functions including drawing the cyclohexane chair and boat conformations, benzene rings and other molecules, see our full guide on How to Use The Pocket Chemist.
Free shipping in the USA through our website or with Amazon Prime!

Want to see all the features of The Pocket Chemist?
---
Do you know someone who loves chemistry?
See our curated collection of Gifts for Chemists and Chemistry Students
Leave a comment
Comments will be approved before showing up.
Author
Derek Miller, Ph.D.,
Materials Scientist and founder of Genius Lab Gear
Gifts Lists by Major
Stay up to date
Drop your email to receive new product launches, subscriber-only discounts and helpful new STEM resources.
Disclosure
As an Amazon Associate I earn from qualifying purchases.

The Ultimate Lab Coat Guide: 5 Types Every Scientist Should Know
5 min read
After spending three years researching lab coats and surveying over 1,500 scientists in what I call "The Lab Coat Project," I've discovered something troubling: most of us are wearing the wrong lab coat. Let me break down the five major classes of lab coats you'll encounter, so you can make an informed decision that keeps you safe and comfortable in the lab.

Do Lab Coats Have to Be White?
4 min read
Ever wondered why lab coats are white? Discover the surprising history behind the iconic garment and why today’s scientists might wear more than just white in the lab.
Recently viewed products
Carbon-neutral shipping on all orders
10253kg
shipping emissions removed
That's like...
26248
miles driven by an average gasoline-powered car
We fund innovations in...
Direct Air Capture
Bio Oil
Mineralization
GET UPDATES
Leave your email to get our monthly resources for scientists, plus new discounts and community projects!



